Alumina with many variations
Release time:
2025-06-25
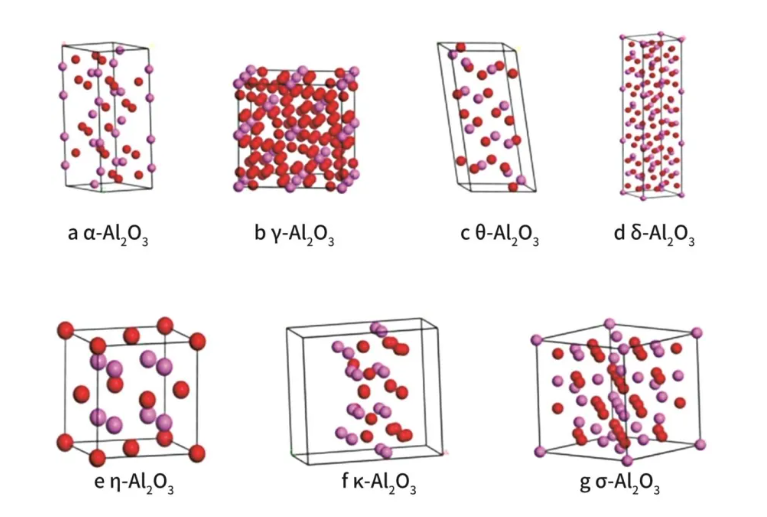
Alumina has a complex crystal structure and has many allotropes, which can be divided into more than a dozen crystal forms, including α, β, γ, δ, ε, ζ, η, θ, κ, λ, ρ, and χ. α-Al2O3 is a stable phase, and the remaining crystal forms are transitional phases, which will transform into the α phase at 1200°C. The conversion process includes the breaking and combination of chemical bonds, which is a lattice reorganization, requires a very large amount of energy, and is an irreversible process.
α-Al2O3
α-Al2O3, commonly known as corundum, belongs to the HCP structure and the trigonal crystal system. Its density is 3.96-4.01g/cm3. It is an inorganic non-metallic material with very stable chemical properties. It has high hardness (Mohs hardness 9), high melting point (2050℃), high strength, good wear resistance, good corrosion resistance and high resistivity. Therefore, α-Al2O3 micropowder is widely used in refractory materials, special ceramics, grinding materials, chemical industry, electronics and other fields. The domestic method for preparing α-Al2O3 mainly adopts the method of direct calcination of industrial alumina. According to the particle size of industrial alumina and different calcination temperatures, the particle size and morphology of active α-Al2O3 are also different.
Main properties of α-Al2O3
At present, α-Al2O3 is widely used in abrasive materials, refractory materials, integrated circuit substrates and structural functional ceramics.
β-Al2O3
β-Al2O3 is not alumina in the traditional sense. It mainly appears in two forms: one is a composite of aluminum oxide and sodium oxide, and the other is a composite of aluminum oxide and magnesium oxide. Their characteristics are that the morphology of their powders is difficult to control.
β-Al2O3 crystals are different from α-Al2O3 crystals in that the oxygen atoms are tightly packed. They have a layered structure, with alkali metal ions or alkaline earth metal ion layers alternately stacked in spinel structural units, while oxygen ions are tightly packed in a cubic manner. Metal ions such as Na+ can diffuse rapidly in this plane layer, so β-Al2O3 crystals can conduct electricity and are an important type of solid electrolyte. Therefore, β-Al2O3 can be used to prepare solid electrolyte diaphragm materials in sodium-sulfur batteries, and can also play an important role in ion conduction and isolation of the positive and negative poles of the battery. In addition, β-Al2O3 can also be used as a refractory material and in the electronic field, such as in electronic watches, cameras, stethoscopes, and pacemakers.
γ-Al2O3
γ-Al2O3 is an important porous material with a large specific surface area, good adsorption function, high chemical reaction activity and good catalytic properties. It is also called activated alumina. Its main use is as an adsorbent, desiccant and catalyst carrier, and is often used in the fields of petrochemicals and environmental protection.
θ-Al2O3
The thermal properties and other properties of θ-Al2O3 are between α-Al2O3 and γ-Al2O3. It is a more stable alumina with almost no water in its structure.
δ-Al2O3
δ-Al2O3 has a small specific surface area and a small pore volume, but its crystal peak is stronger than that of other anhydrous alumina variants. The crystal structure model of δ-Al2O3 comes from the Inorganic Crystal Database (ICSD#40200), which is a tetragonal system with a space group of P-4M2. This material has strong adsorption capacity and catalytic activity, and is often used as an adsorbent, desiccant and catalyst carrier.
η-Al2O3
η-Al2O3 has a pore volume and specific surface area similar to γ-Al2O3, and its surface acidity is stronger than Al2O3. It is mainly used as a catalyst carrier. The crystal structure model of η-Al2O3 comes from the Inorganic Crystal Database (ICSD#28919), which is a cubic crystal system with a space group of FM-3M.
κ-Al2O3
κ-Al2O3 is a type of activated alumina. The crystal structure model of κ-Al2O3 comes from the Inorganic Crystal Database (ICSD#94485), which is an orthorhombic crystal system with a space group of Pna21. It is mainly used as a refractory binder, purifier, adsorbent, etc.
σ-Al2O3
The structure of σ-Al2O3 is similar to that of η-Al2O3. Its crystal structure model comes from the Inorganic Crystal Database (ICSD#69213), which is a cubic crystal system with a space group of FD-3MS.
ρ-Al2O3, χ-Al2O3
ρ-Al2O3 and χ-Al2O3 are amorphous aluminum oxides. They are the only aluminum oxides with hydration and gelling properties among all aluminum oxides. They are also called fast-de-powdering in China. Due to their poor thermal stability, hydration reactions often occur with poor thermal stability.
Due to the large variety of crystal structures of aluminum oxide and the large differences in properties between different variants, it has certain applications in various fields.
The conversion relationship between various crystal forms of aluminum oxide
Hydrated aluminum oxide is the key to the generation of anhydrous aluminum oxide, and the structure and properties of the latter are determined by the structure and characteristics of hydrated aluminum oxide.


















